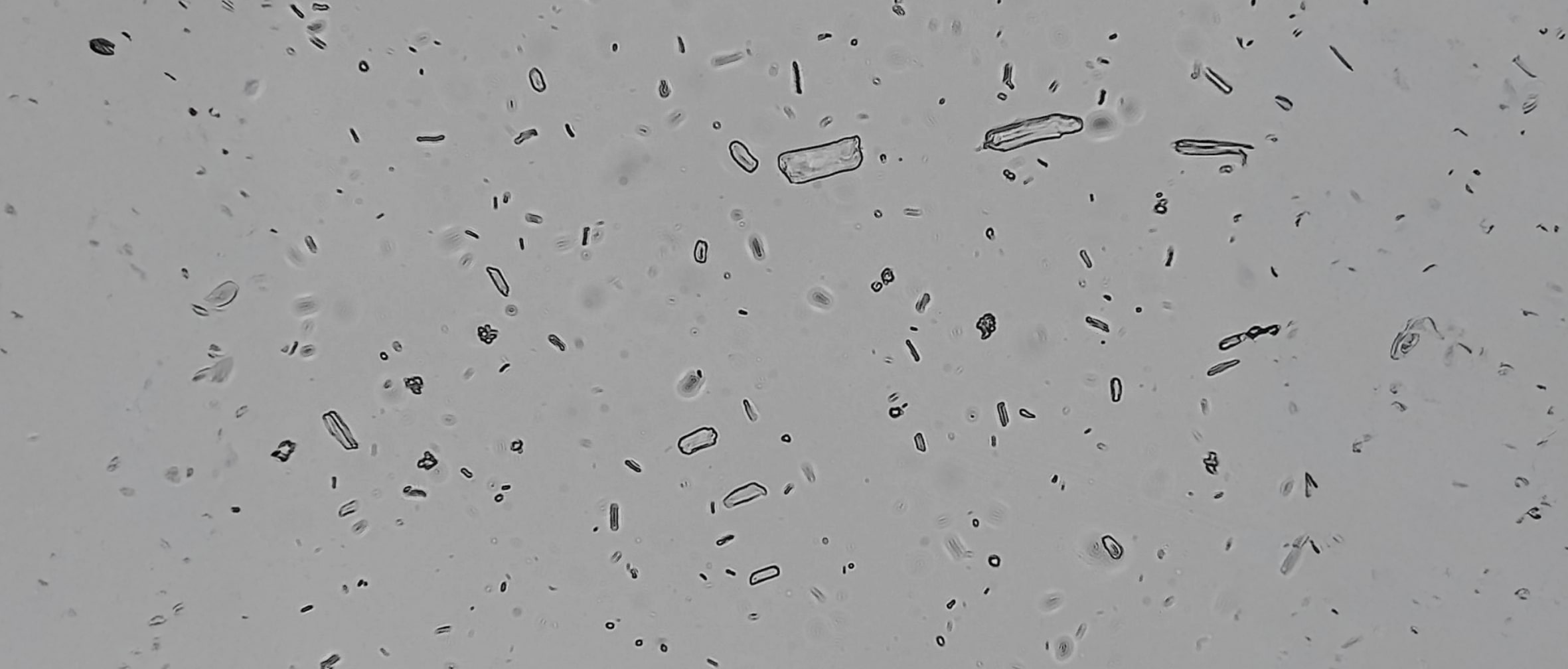
In pharmaceutical R&D, deformulation analysis helps decode the formulation structure to support generic product development. It enables differentiation between APIs and excipients based on particle shape and optical properties. For instance, crystalline drugs show birefringence under polarized light, while many excipients do not.
Low-power optical microscopy can also reveal disintegration patterns—individual particle dispersion in direct compression tablets versus granule formation in wet or dry granulation processes.